11.1 Introduction
A copy of the first edition of Giovanni Battista Benedetti's Diversarum speculationum mathematicarum et physicarum liber exhibits some notes in the margins by Guidobaldo del Monte that were written with iron gall ink. Some of these comments were covered with deletions also carried out with iron gall ink. The study presented here was carried out with the aim to find out if it would be possible to read the original comments underneath the deletions.
The application of band pass filter infrared reflectography technique or fast scan X-ray fluorescence mapping requires a distinct difference between both ink materials. By means of X-ray fluorescence analysis (XRF) we have tried to find out the elemental composition fingerprint to distinguish between both inks.
11.2 Iron gall ink
Iron gall ink is the most used drawing and writing material in Western history.1 In general, it is produced from four basic ingredients: galls, vitriol, gum Arabic as a binding medium and an aqueous medium such as wine, beer or vinegar. By mixing gallic acid with iron sulphate, a water-soluble ferrous gallate complex is formed. Due to its solubility, the ink penetrates the parchment surface, making it difficult to erase. When exposed to oxygen, a ferric gallate pigment is formed. This complex is not water-soluble, which contributes to its indelibility as writing ink. Due to the variety of different recipes2 and the natural origin of different materials, there is a wide range of different components and impurities in historical iron gall inks.
Vitriol, the main source of iron in the iron gall inks, was obtained from different mines and by various techniques.3 Therefore, inks contain many other metals, such as copper, aluminium, zinc and manganese, in addition to the iron sulphate. These metals do not contribute to color formation in the ink solution but possibly change the chemical properties of the inks. The determination of different inorganic components in iron gall inks provides the basis for the differentiation of these writing materials.
11.3 The archaeometric fingerprint method
The chemical composition including trace components is a characteristic property of an art object. As mentioned before the qualitative and quantitative investigation of minor or trace components leads to composition fingerprints which allow to differentiate between varying iron gall inks. This is usually not possible by means of visual examination or with further non-destructive techniques.
For a reliable quantitative analysis of the XRF-data, several technical parameters have to be taken into account. Due to the fact that light elements such as carbon, oxygen and hydrogen, which are not detected with energy dispersive XRF, are the main components of the ink as well as of some inorganic additives to paper, an absolute quantification is not possible. However, the determination of a fingerprint which represents the amount of a detectable trace element in relation to the main compound iron enables the characterisation of different iron gall inks. As mentioned before iron gall inks contain many other metals, such as copper, aluminium, zinc and manganese, in addition to the main inorganic component iron sulphate. The fingerprint method relies on the determination of characteristic elemental compositions in samples.
In the fingerprint model, the ink-paper system is regarded as a three-layer-model-system with a top layer of iron gall ink, a diffusion layer with a linear decreasing amount of ink and a bottom layer consisting of paper. For this system of layers a fundamental parameter approach4 for the primary fluorescence is made. The primary intensity can be expressed as the sum of the contributions from the ink and from the paper. The successive computations take into account all physical phenomena which contribute to the formation of X-ray fluorescence. After all, this leads to the fingerprint value, which depends on three parameters: the transmission of the entire system, the penetration depth of the ink into the paper and the ratio of the absorption coefficients. For all minor constituents i such as copper, aluminium, zinc and manganese a fingerprint value Wi can be specified. This fingerprint value represents the amount of a minor constituent relative to the main compound iron (e.g., WCu = concentration (Cu) / concentration (Fe)).5
11.4 Experimental set-up
Analyses were carried out with the mobile energy dispersive micro-X-ray spectrometer ArtTAX® (formerly Röntec-GmbH, Berlin, Germany, see figure 11.1), which consists of an air-cooled low-power molybdenium tube, polycapillary X-ray optics (measuring spot size 70 µm diameter), an electrothermally cooled Xflash detector, and a CCD camera for sample
positioning. Furthermore, additional open helium purging in the excitation and detection paths enables the determination of light elements
(11 < Z < 20) without vacuum. All measurements are made using a 30 W low-power Mo tube, 45 kV, 600 µA, and with an acquisition time of 25 s (live time) to minimize the risk of damage. For better statistics, at least ten single measurements were averaged for one data point. Further details concerning the method are described elsewhere.6
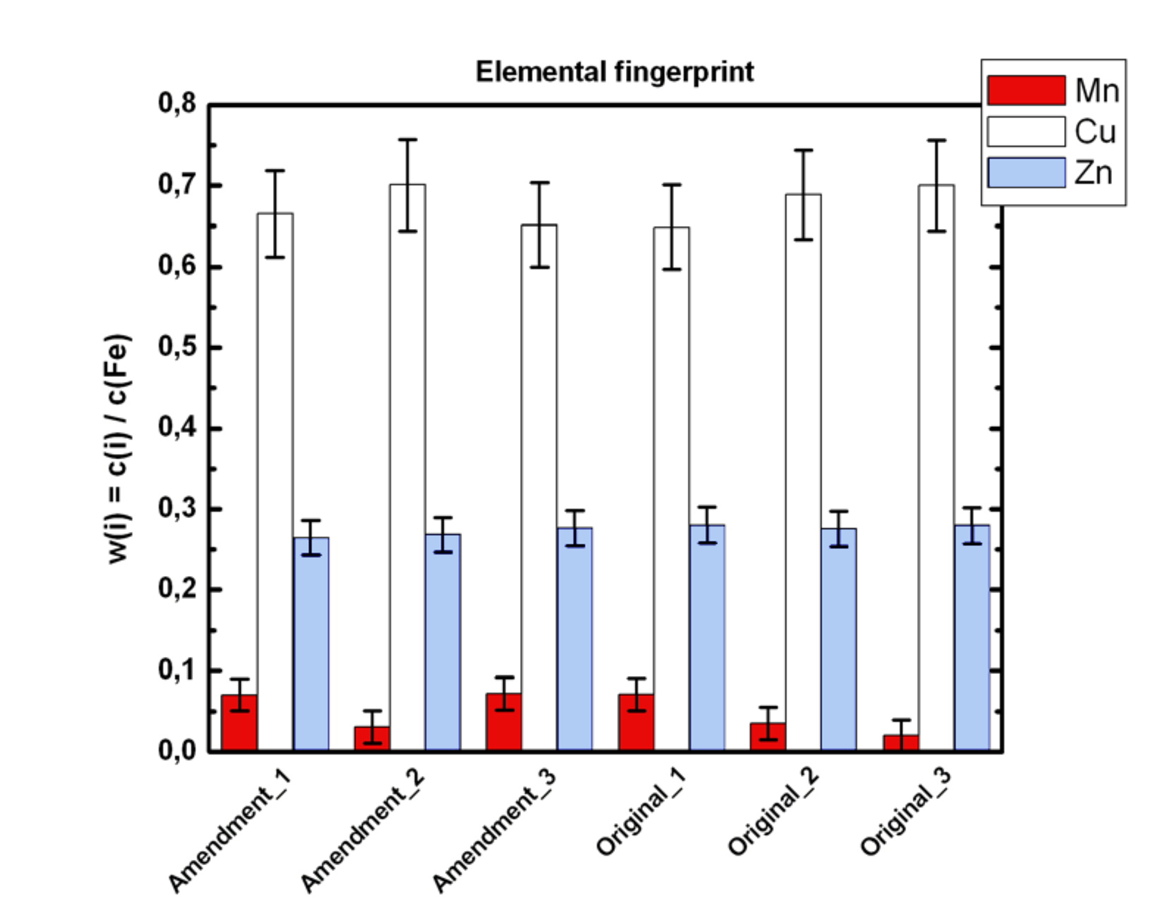
Fig. 11.2: Elemental fingerprints (quantification by means of fundamental parameter approach) of “original” iron gall ink and “deletion” iron gall ink.
11.5 Results
The original iron gall inks as well as the deletion inks have the same elemental composition. Taking into account the measuring faults (see error bars in figure 11.2) it is obvious that there is no difference between “original ink” and “deletion ink.” Due to this result it will not be possible to distinguish between both ink materials. As a further consequence it will not be possible to read the original comments underneath the deletions by use of non-destructive techniques.

